NEWS
Dornier MedTech announces FDA clearance and CE mark approval for the revolutionary Nautilus urology table
- Dornier launches its new flagship urology table, the Nautilus, after receiving FDA clearance and CE mark approval.
- The Dornier Nautilus brings the next level of flexibility, procedural versatility, and radiation management in urology tables.
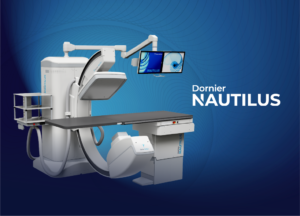
Dornier MedTech (Dornier), a leader in the global urology marketplace, is proud to announce that it has received FDA clearance and CE mark approval for its new Nautilus urology table.
Introducing Urology’s finest with a next-generation urology system
For decades, urology tables have remained virtually unchanged, with only incremental improvements in their imaging systems. Dornier has completely reimagined the urology table, with the patient, urologist, and the hospital in mind. The Nautilus offers new capabilities never before found in a urology table, combining a free-standing motorized urology table and the benefits of a mobile C-arm into one powerful, ergonomic platform. Capable of facilitating a wide range of urology procedures, Dornier’s flagship Nautilus will usher in a new era in table design in the urology treatment space.
Delivering unrivaled flexibility
Dornier MedTech continues its long tradition of innovation in urology by providing best-in-class products to urologists. The Nautilus enables high-resolution imaging from unprecedented angles with a flexible full-ranged C-arm and precise multi-axis table movements, featuring independent movement or sophisticated synchronization. The unique C-arm movement and parking position allows the Nautilus to be used as a free-standing urology treatment table with true 360° table access for endoscopic and surgical procedures. In addition, its X-ray tube can be flexibly positioned below the patient table, enabling enhanced radiation management.
Brock Faulkner, Chief Executive Officer of Dornier MedTech America said:
“Urology tables and their functionalities have not changed significantly in over 30 years, until now. We are confident that our groundbreaking Nautilus will set a new industry standard for urology tables. Its revolutionary design and unrivaled flexibility, coupled with its excellent imaging capabilities, will pave the way for our customers to provide additional urological treatment procedures that will improve the workflow for the urologist and the operating room.”
Wong Yau Chung, Chief Operating Officer at Dornier MedTech added:
“Dornier has spared no effort to develop this product, challenging decades of conventional thinking. The FDA clearance and CE mark approval of the Nautilus is a major milestone in our proud history of game-changing innovation in urology. Our people have poured their passion into designing and bringing to market this product to fulfill the unmet needs of patients, urologists, and hospital administration. We are excited to offer the most innovative urology table on the market and we’re confident it will exceed our customers’ expectations.”
The Dornier Nautilus is now available for order in the United States and the European Union.
Find out more about Nautilus here.
About Dornier MedTech
Dornier MedTech is a medical device company headquartered in Munich, Germany, and is a full subsidiary of Advanced MedTech. As a pioneer in the field of urology, Dornier is one of the most trusted names in the industry.
Dornier commits to drive clinical performance and spearhead innovation in urology, launching several pioneering technologies and revolutionary therapies in recent years. As the world’s first MDR-certified integrated urology company, and one of the original founders of the Urology Care Foundation (formerly known as the American Foundation for Urologic Diseases), it takes pride in holding itself to the highest standards of patient safety and product efficacy.
Today, Dornier is pushing the envelope in digital urology to provide holistic care to patients seeking treatment and community support in their recovery journey, through online and in-person channels.
Trusted by thousands of physicians and patients, Dornier is committed to serving and supporting our urology communities worldwide.
For more information, visit www.dornier.com.